| |
|
|
Clinical Staffing

At Sky-Hi technologies we are also capable of bringing life-changing medicines, therapies, and products to market by using our skills and working effortlessly . In order to do that, you need the best and brightest talent in life sciences working for you. Ski-hi is committed to providing path-breaking and is renowned for setting unique benchmarks in science & technology, research, design, and quality services. We believe you deserve a hiring partner who truly understands your business and who empowers you to hire with confidence.
we offer a full suite of outsourcing, consulting, and staffing solutions.
We will work closely with you to listen to your needs, consult you on the most strategic solution, and partner with you to deliver that solution. So, contact us today, stop settling for subpar talent and instead partner with the industry experts and focus more on what you do best: growing your job in an effort to improve quality of life for communities around the world. We are professional in approach and very accommodative.

we work with the organizations, recruiters, and other resources in order to find the perfect fit for your position or positions. Depending on your clinical hiring needs, like temporary or temp to hire or other positions, our consultant will offer you variously qualified and experienced experts.
- Contract/C2C
- Contract-to-Full-Time
- Full-Time
- Functional services (FSP)

The Clinical process is simplified with our functional moxie for cooperative planning, perpetration and operation.
Clinical operation is really one of the most critical aspects of clinical exploration. It takes a lot of trouble to plan and execute clinical exploration while mollifying pitfalls strictly. Also, with increased regulations and guidelines, it has come precipitously critical to put time and trouble into engaging a clinical operations platoon that can fleetly handle the compliance as well as fulfill all the conditions.
At Sky-Hi Technologies, we offer end-to- end and customized clinical operations services to aggressively supereminent clinical trials for a wide range of medicines around the globe
Monitoring Services
Problem Solving Monitoring Solutions
Successful and smooth execution of a clinical trial requires a lot of effort. There can be so multitudinous impending challenges like slow enrollment, point problems, delayed performance of amendments etc. In fact, point or CRO problems can sometimes unhinge a clinical program. Global Pharma Tek in cooperation with Devoted Point Results provides SWAT/ Deliverance ( study within a trial) monitoring services across the United States in alltimezones.However, covering resources can be trained as per the client’s protocol and on the first point visit within seven days, If demanded.
High- points of our educated and devoted deliverance monitoring team
- Efficiently manage tight timelines
- Attention to critical endpoints
- Proven point connections with KOLs in multiple suggestions
- Nonfictional connections with major healthcare installations and group practices across the United States
- Work singularly or in groups for deliverance monitoring
- Clinical Trial Feasibility
- Clinical Feasibility Services to Asses & Mitigate Research Delays
Being a fundamental member of study set up, feasibility studies include a thorough review of nonsupervisory contemplation and execution of doable ramifications for the success of a clinical disquisition in a specific geology.
Sky-Hi Technologies broad moxie, global presence and regular clinical system help in assessing the methodology and recognizing the most productive and quick conduct to achieve clinical disquisition success. Our ever- adding database can give you recognizable substantiation of new investigators and spots. We also give important and doable data to make the clinical study more effective.
Clinical Project Management
Adaptive, Creative & Agile Clinical Project Administration
Clinical trials have expanded to multiple dimensions and require apt management for its successful accomplishment. But this is not an easy task owing to different complexities and criticalities of the clinical research. Sky-Hi Technologies team of highly experienced and sharpened project managers are masters in managing complex reviews across various therapeutic areas around the globe. Our team is well versed and eager to achieve operational perfection by following best industry practices to deliver on-time and high-quality outcomes.
Our project administration group caters to all the needs of our clients in the key market areas of Asia, Europe and North America.
Medical Monitoring
Medical Monitoring to Stay on Track
Our project administration group caters to our clients’ needs in the key market areas of Asia, Europe, and North America. The teams have a sharpened aptitude and are constantly trained to deliver better outcomes to our clients. They are committed to keeping the project on track while maintaining its quality. Sky-Hi Technologies gives 24×7 support to all the clients for eligibility review and exact randomization of a subject.
Clinical Documentation
Compliant Clinical Documentation System
Clinical documentation is an important part of clinical research. More important, clinical documentation must be compliant to the stringent regulatory standards and regulation. Thus, it is necessary to have a documentation system that guarantees quality documentation according to the regulations and is compliant with ICH-GCP and other standard operating procedures. Sky-Hi Technologies offers a well-planned and robust clinical trial management framework that is compliant with 21 CFR Part 11 and can strengthen archive tracking at the global level.
Flexible Suite of Drug Safety & Pharma covigilance Services
Developing drugs and devices to manage human health is indeed a serious business but developing safe drugs for mankind is the truth of the truths.. Of all the steps, ascertaining the safety of drug is the most crucial one as an unsafe drug can do more harm to the body than the disease itself. So, it becomes most important for the pharmaceutical company to identify the safety information about the drug/device in a timely manner to avoid any future uncertainty. To do so and ensure the safety of underdevelopment drug/device, the most essential step is to thoroughly collect as well as analyse its safety information data. A well collected and analysed data is required for the success of clinical research and to maintain the post-marketing drug licenses.
At Sky-Hi Technologies, we understand the criticality of patient safety, drug safety and pharmacovigilance for every clinical research. We offer drug safety and pharmacovigilance consulting services throughout the life cycle of a drug/device development spanning from preclinical, clinical to post-marketing. Our team of experienced drug safety and pharmacovigilance experts can assist you in evaluating and managing the safety concerns of your product.
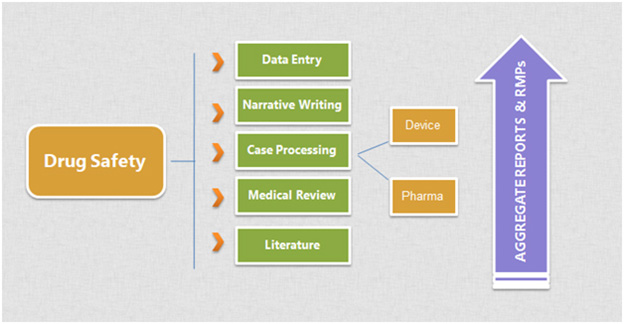
End-to-end Drug Safety and Pharmacovigilance Solutions
Sky-Hi Technologies is an emerging CRO offering end-to-end solutions in drug safety and pharmacovigilance. Our globally scattered team is focused to deliver accurate, compliant and effective pre- and post-approval pharmacovigilance services. We offer an extensive range of services in safety and risk management including
- State of the art call centres
- Case preparing and processing
- Well-being reports of individuals
- Total safety detailing reports
- Safety signal detection
- Risk administration and management
- Counselling for pre-advertise improvement and post-marketing exercises
Global Team of Pharmacovigilance Experts
Our large globally expanded team of safety and pharmacovigilance experts possess strong academic qualifications include medical doctors, PV physicians, pharmacists, medical writers, life science postgraduates and Ph.Ds. Each of our team member is well experienced and skilled to seamlessly deliver solutions for all your safety and pharmacovigilance concerns. With growing industry experience, our team has gained deep knowledge of global processes and legislation.
Compliant and High-quality Pharmacovigilance Services
Our thorough understanding and apt implementation of relevant guidelines and regulations aim to help all our clients in meeting the strict regulatory requirements. Our staff is strategically placed to support specific regulatory requirement of location. Our attention to consistency to yield the maximum product potential while strictly taking care of patient safety.
Customized Solutions
As no two clients can ever be same, their requirements can also not be same. So, our efforts are always concentrated to build a relationship of trust and offer personalized and tailored services to all our clients. We provide specific and customer centric solutions according to the requirement of our client. Our team can swiftly adjust in any requirement and can efficiently work for individual activities as well as for whole pharmacovigilance system.
Seamless Delivery Solutions
We focus to bring a seamless delivery of solutions for all our services while maintaining transparency, compliance, quality and operational efficiency.
Always Up-to-date with Regulatory Changes
Regulations and guidelines for drug development, patient safety, drug safety and marketing keep on evolving and it is a challenge for a pharma company to keep a track on all these changes and work accordingly. Moreover, with every change in regulations, the pharmacovigilance legislation becomes more stringent that dramatically changes the compliance scenario. Sky-Hi Technologies helps its clients in following the updated guidelines with preparation and submission of more specialized and robust reports for quick and hassle-free approvals.
Dedicated Subject Matter Experts
Apart from a team of highly experienced and skilled team of safety and pharmacovigilance experts, our dedicated and skilled subject matter experts in safety regulatory submissions and legislation ensure accurate and compliant pre- and post-approval pharmacovigilance globally. Hence, outsource your safety and pharmacovigilance project to us ensure on-time safety reporting for prompt actions.
Highest Level of Safety & Pharmacovigilance Services
Our experienced and dedicated team of pharmacovigilance experts work very hard to submit all reports well within time and client’s budget. Our quality results are a boon for client and help them in early product approval.

Solution for your regulatory challenges
Regulatory Services for Complete Clinical Continuum
The clinical journey of a new product is often difficult and lengthy. It involves preclinical development, clinical trials, regulatory submissions and finally regulatory approval to market the product. The most tedious and stringent of all these is regulatory affairs. Regulatory bodies have become stricter than ever before and are constantly evolving guidelines and regulations for the betterment of mankind. At Sky-Hi Technologies, we understand the nuances of complex regulatory requirements and offer regulatory support to Pharma and Biotech companies at every stage of their clinical development. Our Regulatory Affairs (RA) department in co-operation with our international network of regulatory affairs professionals, facilitate regulatory solutions for drug or device at every stage.
We provide regulatory advice, solutions and support for every regulatory requirement.
Our Global Regulatory Services Include:
- Drug Substance Services: DMF, IND, Manufacturing, Import & Exports
- Drug Product Services: NDA, ANDA
- Pre-IND and Pre-NDA meeting with FDA
- Writing and compilation of INDs, NDAs, ANDAs, 505(b) (2) and supplements
- eCTD submission
- Providing the clearances
- Orphan Drug Submissions
- Regulatory support for clinical trials, Facility Inspections
- CMC (Chemistry, Manufacturing, Control) and Pre-Clinical toxicology
- Regulatory agency interaction
- Case Report Forms (CRFs)
- Product information (SPC, PILs)
- RLD Sample Procurement
- Pricing and Market Authorization Approval
- Product Life Cycle Management
Drug Master File - DMF
DMC is an important technical document of the registration dossier and contains CMC (chemistry, manufacturing & control) information about the active pharmaceutical ingredient.
Sky-Hi Technologies Offers the following Drug Master File services:
- DMF compilation in CTD Format / Country Specific format
- Open & close part DMF writing
- Review of DMF for Submission
- Type II (Active Ingredient) DMF Preparation and submission to US FDA
- European Certificate of Suitability (CEP) submission and Preparation
- Canada Submission & Preparation
- Analytical Testing – Elemental Analysis, Validations, Stability, etc.
CTD - Dossiers
One of the most important parts of any registration application for Marketing Authorization is Common Technical Document (Product Dossier). It needs to be compiled in CTD Format/ ACTD Format or local country format for its submission to Food & Drug Authority (FDA) or Ministry of health (MOH) or any other equivalent authority. In addition to CTD, other technical documents and legal manufacturing permissions are also required for market authorization. At Sky-Hi Technologies, we understand the complexity of this task and can help you prepare the entire registration file for drug product registration around the globe.
Sky-Hi Technologies offers following Dossier Compilation services:
Dossier writing and compilation as per CTD Format - Common technical Document (for regulated and semi regulated market like Middle Eastern countries, CIS Countries, European Union, Canada, Australia, USA, Japan, etc.)
- Module 1 – Administrative Information
- Module 2 – CTD Overview
- Module 3 – Drug & Product Part /CMC
- Module 4 – Non-Clinical
- Module 5 – Clinical
Dossier writing and compilation as per ACTD Format – Asian Common Technical Document (for Asian Countries like Thailand, Vietnam, Malaysia, Singapore etc.)
- Part I – Administrative Documents
- Part II – Quality Documents
- Part III – Non-Clinical Documents
- Part IV – Clinical Documents
Highly Skilled & Flexible Regulatory Affairs Team
Our interdisciplinary, expert and supportive regulatory team members are flexible to take care of your complete regulatory responsibility or offer you a program especially tailored to your needs. We can help you compile and write any specific module or the entire technical document as per your need. In addition to this, we also offer support services to companies in establishing their Document Management and technical writing system. We help them in drafting templates on BMR, COA, MOA, and other technical documents.
Our regulatory affairs team aims to ensure the preparation, submission, and approval of regulatory compliant documents for every clinical trial stage. We work in close collaboration with the client’s team to ensure quality, global compliance, consistency and support at every stage.
Our full-service or customized regulatory solutions cater to the needs of every company- Good for both Start-up and scale up companies.
With years of combined experience and understanding of pharmaceuticals, biotechnology and medical device industries, Sky-Hi Technologies provides best-in-class staffing and recruiting support to all validation projects, big and small. Our validation team understands the extreme importance of ensuring the reliability and integrity of business information and delivers top talent that takes your projects through completion.
Our team can assist in finding best-in-class talent within the following functional areas:
- Computer Systems Validation
- Equipment Validation
- Process Validation
- Cleaning Validation
Below is a snapshot of searches we assisted:
- IT CSV Manager
- Validation Lead
- QA Lead (QMS)
- Validation Analyst
- Data Integrity Lead
- Validation Engineer
- Qualification Engineer
- Process Engineer
|
|
|
|
|